Influence of backbone substituents on the ethylene (co) polymerization properties of α-diimine Pd (II) and Ni (II) catalysts
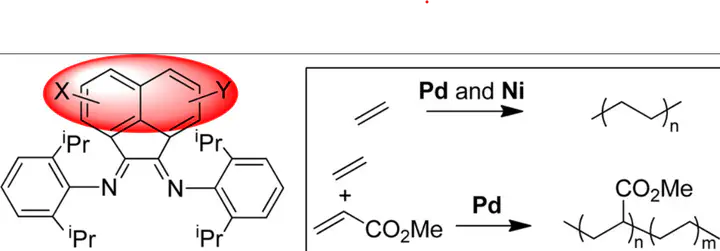 Image credit: Unsplash
Image credit: UnsplashAbstract
A series of α-diimine ligands with different substituents on the acenaphthyl backbone were synthesized and characterized. The corresponding Ni(II) and Pd(II) complexes were prepared and used in ethylene polymerization and copolymerization with methyl acrylate. In ethylene polymerization, these Ni(II) complexes showed activities of up to 1.6 × 107 g/((mol of Ni) h), generating polyethylene with a molecular weight (Mn) of up to 4.2 × 105. Interestingly, these Ni(II) complexes behave very similarly in ethylene polymerization except for the complex with two methoxy substituents on the ortho position of the acenaphthyl backbone, in which case about 3 times higher polyethylene molecular weight and much lower branching density were observed. The ligand substituent effect is much more dramatic for the Pd(II) complexes. In ethylene polymerization, activities of up to 1.7 × 105 g/((mol of Pd) h) and a polyethylene molecular weight (Mn) of up to 4.7 × 104 could be obtained. The Pd(II) complex with two methoxy substituents on the ortho position of the acenaphthyl backbone demonstrated much higher activity and generated polyethylene with about 3 times higher molecular weight than that for the classic Pd(II) complex. A similar trend was maintained in ethylene–methyl acrylate copolymerization..
Add the publication’s full text or supplementary notes here. You can use rich formatting such as including code, math, and images.